Current Situation Taken From CDC as of October 9, 2012
- CDC is aware that New England Compounding Center (NECC) has voluntarily expanded its recall to include all products currently in circulation that were compounded at and distributed from its facility in Framingham, Massachusetts.
- Clinicians should continue to contact patients who have received medicines associated withthree lots of preservative-free methylprednisolone acetate (80mg/ml) recalled on September 26. The potentially contaminated injections were given starting May 21, 2012. See: Clinician Guidance
- CDC’s guidance to patients has not changed as a result of this voluntary recall. Patients who feel ill and are concerned about whether they received a medication from NECC at one of the affected facilities should contact their physicians.
- Patients have had symptoms generally starting from 1 to 4 weeks after their injection.Not all patients who received the medicine will become sick. Symptoms that should prompt patients to seek medical care include: fever, new or worsening headache, neck stiffness, sensitivity to light, new weakness or numbness, increasing pain, redness or swelling of the injection site. See: Patient Guidance
Persons by State with Meningitis Linked to Epidural Steroid Injections,
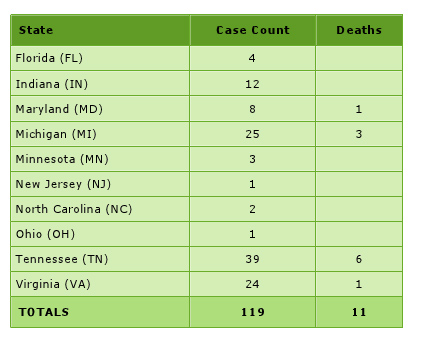
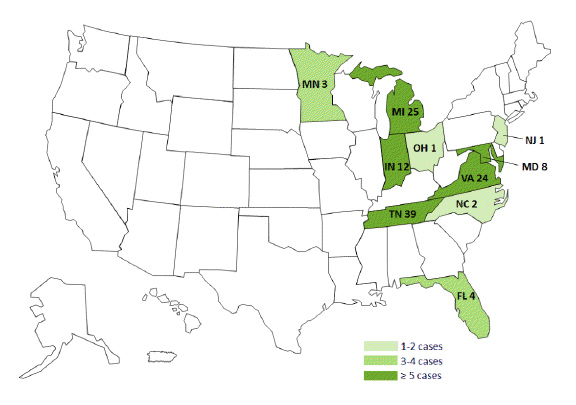
For more information, go to: http://www.cdc.gov/HAI/outbreaks/meningitis.html
